Human Serum Paraoxonase 1 (PON1) is a calcium dependent enzyme capable to hydrolyze paraoxon i.e. a metabolite of insecticide parathion. The name ‘paraoxonase’ is given due to its native activity to hydrolyze paraoxon. Later on it was identified that it can hydrolyze many lactones, esters, drugs and some other pesticide metabolites.
PON1 is a glycosylated protein consisting of 355 amino acid residues with a mass of 43-47 kDa. It is synthesized in the liver, and then secreted in to blood where it is mainly bound to high density lipoproteins (HDL). The crystal structure of PON1 has not been identified yet but a crystal structure of recombinant PON1 (rePON1) derived from rabbit PON1 was reported in 2004. This three-dimensional structure of a hybrid mammalian rePON1 variant was obtained by directed evolution of four mammalian species (mouse, rabbit, rat, humans). The recombinant structure has elucidated over all architecture of PON1 explaining the dynamics of PON1. RePON1 structure consists of six bladed β-propeller having a phosphate group and two calcium ions in the center of propeller that play a vital role in maintaining the stability of the structure. Figure 1 displays the structure of Human PON1 that has been designed through Homology Modeling.
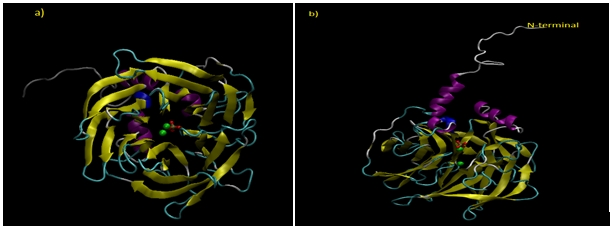
Figure 1: Structure of Human PON1 (Predicted by Homology Modeling). a) Six bladed β-propeller structure of PON1. Two calcium ions are represented in green color while phosphate group is shown in red. b) A side view of PON1 indicating N-terminal hydrophobic region which helps in attachment of PON1 to HDL particles.
Function 0f PON1
Up till now the exact function of PON1 is still not identified that may be due to reason that its true physiological substrate is unknown but is thought that enzyme has a protective influence against neurotoxicity of organophosphates and lipid oxidation. Early studies reported that species with high levels of PON1 are much more resistant to organophosphates (OPs) than those having low levels of PON1. In comparison of selective toxicity of PON1 in birds and mammals, birds were found to be more prone to OP poisoning than mammals because of very low PON1 level in birds. In vitro studies reveal that injecting the rabbit PON1 into rats make them more resistant to paraoxon and chlorpyrifos oxon.
PON1 Polymorphisms
More than 200 single nucleotide polymorphisms (SNP) have been identified in human PON1 gene in coding and non-coding region but two polymorphism at 55 and 192 positions in Human PON1 protein sequence are considered to affect its concentration and activity. At 192 glutamine (Q) is transformed to arginine (R) where as Leucine (L) is replaced by Methionine (M) at 55 position. These two isoforms of the enzyme differ greatly in the rate of hydrolysis with number of substrates. Individual having MM and QQ genotypes at 55 and 192 positions respectively are considered to be more protective against oxidation of Low Density Lipoproteins (LDL) while LL and RR are more vulnerable for oxidative stress. Similarly LL and RR have greater paraoxon hydrolytic activity while MM and QQ have least paraoxonase activity. Therefore, database is developed that shows the global distribution of PON1 SNPs in normal population as well as investigating the different reported diseases related to PON1 polymorphisms.
Database For PON1 Polymorphism
Distribution of PON1 SNP in healthy individuals is different from country to country possibly because of difference in ethnicity and geographic isolation of populations. Different countries show different allelic distribution of L/M at 55 and Q/R at 192. Figure 2 highlights those countries for which data was collected for the normal allelic distribution of PON1 polymorphisms.
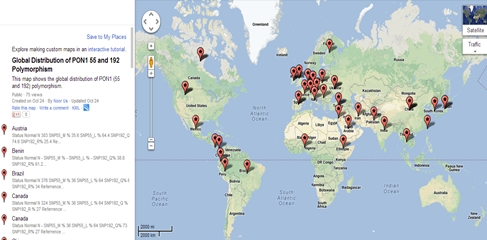
Distribution of PON1 SNP in healthy individuals is different from country to country possibly because of difference in ethnicity and geographic isolation of populations. Different countries show different allelic distribution of L/M at 55 and Q/R at 192. Figure 2 highlights those countries for which data was collected for the normal allelic distribution of PON1 polymorphisms.
Figure 3 is the snapshot of database displaying the allelic frequency of PON1 polymorphism at 55 and 192 in Panama.
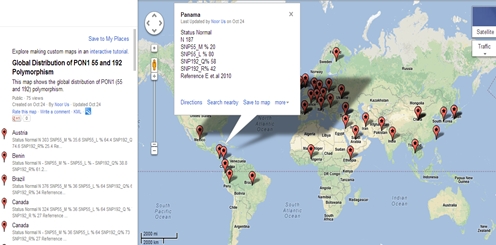
Figure 3: Allelic distribution of PON1 polymorphism in Panama.
Name of the country, allelic frequency distribution of L/M and Q/R at 55 and 192 position of PON1, reference from where the particular data was taken is displayed. N is the number of subjects that are recruited for a particular study
Database link for PON1 polymorphisms in different countries
Link:
Atherosclerosis or Cardiovascular diseases (CVD) and Diabetes Mellitus (DM) are mostly associated with PON1 and its polymorphisms. Figure 4 displays the countries for which diseases related data of PON1 polymorphisms was gathered.
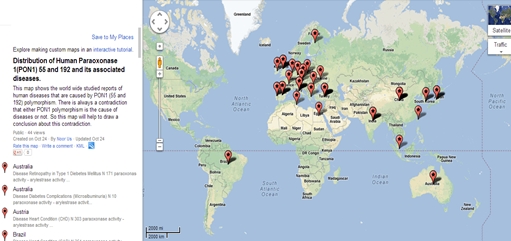
Figure 4: PON1 polymorphisms and its association with different diseases. Countries are represented by red mark for which data was collected.
Figure 5 is the snapshot of database displaying the association of PON1 polymorphism with Coronary Heart Diseases (CHD).
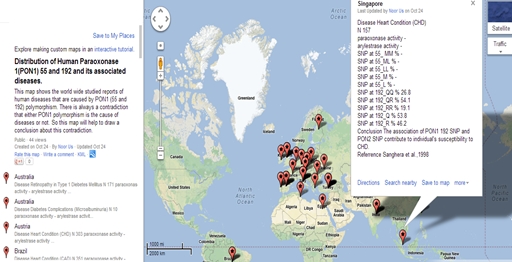
Figure 5: Frequency of PON1 polymorphism in patients suffering from CHD in Singapore.
Name of the country, allelic frequency distribution of L/M and Q/R at 55 and 192 position of PON1, genotypic frequency distribution at 55 and 192 position, reference from where the particular data was taken is displayed. N is the number of subjects that are recruited for a particular study.
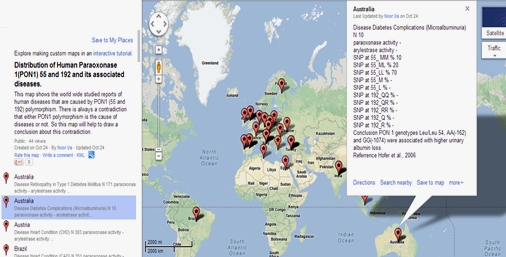
Figure 6 : Frequency distribution of PON1 polymorphism in Microalbuminuria in Australian population.
Database link for detail study of PON1 polymorphism and its association with different diseases
Link:View Details
Contributors
This database was developed as a part of final year project from the students of BS-Bioinformatics for particular research on PON1 polymorphism and its association with different human diseases. The particular study was conducted under the supervision of Dr. Habib Bokhari, the chairperson of Bio-Sciences department of Comsats Institute of Information Technology (CIIT), Islamabad, Pakistan. Dr. Habib has done his PhD in Microbiology/Immunology from University of Glasgow and subsequently accomplished his Post-Doc from Virginia and London. His areas of research are Infectious Diseases, Glycoengineering, Biosensors and Bioinformatics.
Noor us Sehar and Khadeeja Siddique are graduating students of CIIT, working on global distribution of PON1 polymorphism and its associated diseases. The aim of their study was to search the potential alleles of PON1 that are more prone to a certain disease. In their research they also correlated the associated diseases of PON1 polymorphism with different demographic factors.
For any query regarding this database, please contact noor.khadeeja@gmail.com.
References
Costa, L. G., Cole, T. B., Jarvik, G. P., & Furlong, C. E. (2003). Functional genomic of paraoxonase (PON1) polymorphism: Effect on pesticide sensitivity, cardiovascular disease, and drug metabolism. Annu. Rev. Med , 54, 371–92.
Costa, L. G., Cole, T. B., & Furlong, C. E. (2005). Paraoxonase (PON1): From Toxicology to cardiovascular medicine. Acta Biomed. , 50-57.
Hegele, R. A. (1999). Paraoxonase genes and disease. Ann Med , 31, 217-224.
Himbergen, T. v., Tits, L. v., Roest, M., & Stalenhoef, A. (2006). The story of PO N1: how an organophosphatehydrolysing enzyme is becoming a player in cardiovascular medicine. The Journal of Medicine , 64, 34-38
Mackness, B., Durrington, P. N., & Mackness, M. I. (1998). Human Serum Paraoxonase. Gen. Pharmac , 31 (3), 329-336.